artículos
Los daguerrotipos de Mathew Brady en el Instituto de Valencia de Don Juan
Objetivo: analizar los daguerrotipos del prestigioso fotógrafo estadounidense Mathew Brady que se conservan en el Instituto de Valencia de Don Juan de Madrid, con el fin de conocer su contenido, determinar sus características formales y poner en valor su importancia para la historia de la fotografía.
Metodología: análisis de forma y fondo de cada uno de los cinco daguerrotipos de Brady, mediante la elaboración de una ficha descriptiva ad hoc a partir de las normas archivísticas ISAD(G), de modo que sea factible la recuperación de la información de los contenidos y de las características formales. Se ha realizado la reproducción fotográfica de los daguerrotipos para este estudio y se han generado las imágenes digitales que lo ilustran.
Resultados: se contemplan desde cinco aspectos: la autoría, el artefacto, el contenido, el significado sociocultural y la institución. Con respecto al primero, Mathew Brady es uno de los grandes autores de la historia de la fotografía; en cuanto al objeto, los cinco daguerrotipos son considerados obras de arte y piezas únicas de museo por su calidad; el contenido es excepcional al tratarse de retratos infantiles del mismo personaje (Guillermo de Osma), tema con escasa representación en la fotografía de la época; el significado sociocultural queda de manifiesto por ser los primeros modelos fotográficos en la historia de la fotografía y por aumentar considerablemente las obras de Brady registradas en Europa (dos en el repositorio de Europeana, y doce en el portal Daguerrobase); por último, el Instituto de Valencia de Don Juan pasa a ser el centro con mayor número de daguerrotipos de Brady en Europa.
Descripción automática de archivos audiovisuales: NeuralTalk, un modelo de video2text aplicado al archivo de RTVE
Objetivo: determinar la madurez de los sistemas de video-to-text para la descripción automática de imágenes en un archivo de televisión.
Metodología: se realiza una prueba de concepto mediante un sistema de video-to-text desarrollado ad hoc. La prueba se articuló en tres fases o iteraciones distintas entre junio de 2016 y enero de 2017. En las dos primeras iteraciones el sistema analizó un número determinado de contenidos procedentes del archivo de RTVE, las descripciones se valoraron para establecer la tasa de acierto del sistema o, en otras palabras, cómo de cercana era dicha descripción a la que podía haber suministrado un ser humano. En una tercera fase, y previamente al análisis de los contenidos, se entrenó al sistema utilizando técnicas de aprendizaje profundo con el objetivo de mejorar los resultados.
Resultados: los resultados obtenidos ponen de manifiesto que se trata de una tecnología prometedora, si bien resulta fundamental profundizar más en los mecanismos que serían necesarios para su puesta en producción en los archivos de televisión.
La evaluación de la investigación en humanidades en AQU Catalunya
En este artículo se presenta la experiencia práctica de evaluación de la investigación en el ámbito de humanidades que realiza la Agència per a la Qualitat del Sistema Universitari de Catalunya de las persones candidatas a ser profesores universitarios que solicitan una acreditación previa de su trayectoria académica. El objetivo del artículo es explicar las características de estos procesos de acreditación, haciendo énfasis especial en el caso concreto de las disciplinas de carácter humanístico, en las que se observan elementos singulares respecto otros ámbitos científicos que añaden una mayor complejidad. En este sentido, al ser las evaluaciones indirectas, a partir de los indicios de calidad de las publicaciones científicas donde se publica la investigación, esto condiciona enormemente los procesos de evaluación. Así, teniendo en cuenta las recientes aportaciones metodológicas sobre la evaluación de la investigación, como por ejemplo el Manifiesto de Leiden o la Declaración de San Francisco, se concluye la necesidad de evitar aproximaciones meramente cuantitativas, de forma que la evaluación sea respetuosa con la comunidad de investigadores en humanidades, que, por otro lado, debate la mejor manera de abordar la evaluación de su actividad de investigación.
Marc, Maria y David: el diseño de experiencia de usuario (UX) aplicado a la biblioteca pública
Objetivos: desde la perspectiva de diseño de experiencia de usuario (UX), construimos tres usuarios-persona para ayudar a diseñar productos y servicios, digitales o presenciales, y en la tarea de planificar las bibliotecas públicas de Cataluña con el fin último de fidelizar y atraer usuarios y usuarias.
Metodología: una encuesta representativa de la población catalana (1.205 respuestas) y un análisis estadístico bivariante permiten identificar los tres públicos objetivos que, gracias al análisis de contenido de tres grupos focales posteriores, dan lugar a los tres usuarios-persona. La técnica de usuario-persona visibiliza los posibles usuarios a partir de datos reales, facilita la generación de empatía entre el personal de la biblioteca y los usuarios, y asegura que el diseño esté centrado en las personas.
Resultados: los tres usuarios-persona resultantes se describen y se complementan con escenarios basados en evidencias que deben ayudar a diseñar experiencias de usuarios. Estos son, Marc (usuario), estudiante adolescente que pretende codiseñar la biblioteca y que reclama actividades sociales presenciales; Maria (nunca usuaria), jubilada que necesita calor humano y que pone en evidencia la necesidad de una colaboración más estrecha entre servicios sociales y bibliotecas, y David (exusuario), trabajador de más de treinta años que considera que no necesita la biblioteca, aunque desconoce la mayoría de servicios que actualmente le ofrece.
Perspectivas de la ciencia abierta. Un estado de la cuestión para una política nacional en Colombia
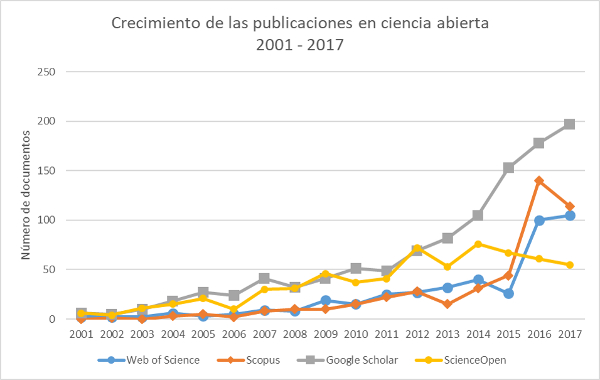
Objetivo: realizar un estado de la cuestión sobre el tema de la ciencia abierta a nivel mundial, para lograr un marco teórico-conceptual amplio, que sirva de base para las recomendaciones generales (retos y perspectivas) para una política nacional de ciencia abierta en Colombia.
Metodología: revisión sistemática de la literatura sobre el tema, en diferentes fuentes de información, tanto abiertas como cerradas, y análisis de contenido para la selección de los aportes más significativos y la elaboración de nuevos aportes teórico-conceptuales al tema. –Resultados: en general, se logra hacer un marco teórico-conceptual sobre la ciencia abierta y la identificación de unos retos y perspectivas para el caso de la construcción de una política nacional de ciencia abierta. Específicamente, como aportes teórico-conceptuales de este estudio al tema de la ciencia abierta para el ámbito iberoamericano y mundial, se logra: 1) definición integradora, construida con base en diferentes y significativos aportes internacionales; 2) taxonomía de la ciencia abierta, traducida al español; 3) línea del tiempo de la ciencia abierta en las últimas tres décadas; y 4) identificación de la situación de la ciencia abierta en lo relativo a políticas nacionales o casos-proyectos concretos destacados en determinados países.
La calidad de las revistas científicas para el profesorado universitario de ciencias del deporte
Objetivos: conocer la opinión del personal docente e investigador de Ciencias de la Actividad Física y del Deporte sobre: a) criterios y productos de evaluación de artículos científicos mejor valorados, y b) las revistas nacionales e internacionales más prestigiosas. La muestra estuvo formada por ciento sesenta y tres profesores universitarios que daban clase en el grado de Ciencias de la Actividad Física y del Deporte en el curso 2013–2014, de los cuales, veinticuatro son evaluadores de alguna agencia de evaluación y ciento treinta y nueve profesores no forman parte de ninguna agencia de evaluación.
Metodología: el instrumento utilizado fue el apartado de artículos y revistas del
Visibilidad de las bibliotecas públicas y la lectura en medios de comunicación españoles frente a otros hechos de la cultura y relación con su uso: medidas para su mayor promoción y difusión
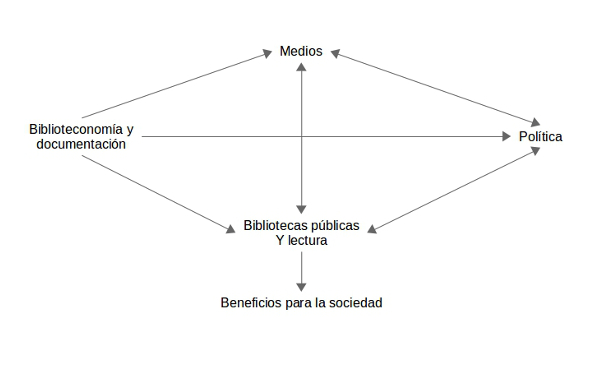
Objectius: el primer objectiu consisteix a analitzar la presència de les biblioteques públiques i la lectura en els mitjans de comunicació espanyols davant d’altres fets de la cultura com el cinema, els museus i el teatre; el segon consisteix a relacionar aquesta visibilitat amb els resultats sobre l’ús de les biblioteques públiques (visites) i l’ús de la col·lecció (préstecs). A partir d’això, en la secció per a la discussió, es desenvolupen propostes per augmentar la promoció i la difusió de les biblioteques públiques per l’increment dels beneficis que poden aportar a la societat. Això es porta a terme mitjançant una reflexió sobre la relació entre els camps de les biblioteques públiques i la lectura, la biblioteconomia i la documentació, la política, i els mitjans de comunicació.
Metodologia: en relació amb el primer objectiu, es fa servir la base de dades MyNews per obtenir el nombre de notícies dels fets culturals que s’han pres en consideració, i es posa en relació amb el total dels fets culturals com a unitats físiques. Pel que fa al segon objectiu, s’estudia la relació entre el total de notícies per 50.000 habitants per a les comunitats autònomes i les visites i els préstecs per habitant a les biblioteques públiques. L’anàlisi d’aquesta relació es fa tenint en compte els resultats de les comunitats autònomes per a les variables i calculant el coeficient de correlació lineal entre aquestes.
Resultats: es constata que la visibilitat de les biblioteques públiques i la lectura davant dels altres fets de la cultura és petita, fins al punt de ser pràcticament inexistent, tot i que hi ha més biblioteques públiques que cinemes, museus i teatres. Al seu torn, es percep una tendència i una relació positiva en les comunitats autònomes entre més visibilitat de les biblioteques públiques i la lectura en els mitjans de comunicació i més quantitat de préstecs i de visites. A partir d’aquesta constatació, es reflexiona entorn de la idea de la retroalimentació positiva entre els eixos de biblioteconomia i documentació, biblioteques públiques i lectura, mitjans de comunicació i política, com a via per incrementar la promoció i la difusió de les biblioteques públiques i per augmentar els beneficis en la societat que es deriven del seu ús.
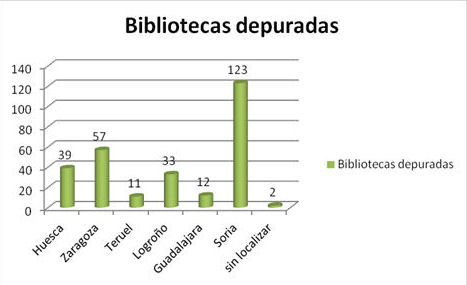
La Comisión Depuradora de Bibliotecas del Distrito Universitario de Zaragoza durante la Guerra Civil (1936–1939)
Objetivo: analizar el significado y la transcendencia de la Comisión Depuradora de Bibliotecas del Distrito Universitario de Zaragoza durante la Guerra Civil (1936–1939), el contexto que impulsó su nacimiento, la procedencia de sus integrantes y su repercusión sobre los servicios ordinarios de la Biblioteca de la Universidad de Zaragoza.
Metodología: análisis de fuentes bibliográficas, hemerográficas y documentales vinculadas al tema.
Resultados: comprensión del significado, alcance y contenidos de la Comisión de Zaragoza.
Del templo simbólico a la desmaterialización: un recorrido por la arquitectura bibliotecaria del siglo XX al XXI
[Versió catalana] Daniel Gil Solés Coordinador técnico Biblioteca Pública Episcopal del Seminario de Barcelona dgil@bibliotecaepiscopalbcn.org Resumen El artículo tiene como objetivo establecer una evolución en la arquitectura de las bibliotecas a lo largo de los siglos xx y xxi. Esta evolución pone de manifiesto las diferentes transformaciones que han sufrido los edificios de … Leer más